Proteins are large, complex molecules that play many critical roles in the body. They do most of the work in cells and are required for the structure, function, and regulation of the body’s tissues and organs.
Proteins are made up of hundreds or thousands of smaller units called amino acids, which are attached to one another in long chains. There are 20 different types of amino acids that can be combined to make a protein. The sequence of amino acids determines each protein’s unique 3-dimensional structure and its specific function.

Protein Structure
|
|
Primary Structure |
- The unique sequence of amino acids that makes up a protein or polypeptide chain.
- Peptide bonds are created by enzyme-catalyzed condensation reactions and broken down by enzyme-catalyzed hydrolysis reactions.
- Breaking down proteins is important in many areas of the body.
- For example, in hormone regulation, cells that are targeted by hormones contain enzymes to break down those hormones. This stops their effects from being permanent and allows them to be controlled.
|
Secondary Structure
|
- Alpha Helices and Beta Pleated Sheets.
- Held together by many hydrogen bonds, overall giving the shape great stability.
|
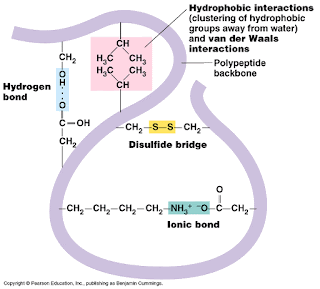
Tertiary Structure
|
- Ø The final 3D structure of a protein, entailing the shaping of a secondary structure.
- Ø Held together by four different bonds and interactions:
- · Disulphide Bonds
- · Ionic Bonds
- · Hydrogen Bonds Hydrophobic and Hydrophilic Interactions
- Ø Tertiary structure can be broken by the action of heat. When a protein loses its shape in this way it is said to be denatured.
|
Quaternary Structure
|
Ø The structure formed when two or more polypeptide chains join together, sometimes with an inorganic component, to form a protein.
Ø The structure is maintained by hydrogen bonds, ionic bonds, hydrophobic interactions, and van der Waals interaction
|
https://www.youtube.com/watch?v=hok2hyED9go
https://alevelnotes.com/notes/biology/biological-molecules/biological-molecules/protein-structure
https://www.dreamstime.com/levels-protein-structure-amino-acids-to-complex-molecule-polymer%C2%A0polypeptide-formed-sequences-primary-secondary-image150715433